Targeting the right patient population for primary prevention: the case of diabetes mellitus
Aspirin in the primary prevention of cardiovascular disease in people with diabetes mellitus.
Atherosclerotic cardiovascular disease (ASCVD) is a major complication for people living with type 1 and type 2 diabetes mellitus (DM) and an area of unmet clinical need. Even with tighter control of hyperglycaemia and the reduction of other cardiovascular risk factors people with DM have around a two-fold higher incidence of death and hospitalisation from ASCVD than matched non-DM individuals.
In their review article Rocca and Patrono examine the pathophysiological, pharmacological and clinical evidence that supports low-dose aspirin use in appropriate individuals with DM and place this evidence within the current background multifactorial approach to the primary prevention of ASCVD in people with DM.
DM and pre-DM is characterised by vascular and haemostatic abnormalities such as endothelial dysfunction, platelet activation, low-grade inflammation and oxidative stress which result in an accelerated atherosclerotic status and increased risk of thrombotic complications such as acute coronary syndrome (ACS), myocardial infarction (MI) and stroke.
A reduced responsiveness to standard low-dose aspirin has been reported to occur in around a third of people with DM and this may be due to obesity and increased platelet turnover. Different dosing regimens are therefore being investigated. People with DM also seem to have a reduced responsiveness to clopidogrel and prasugrel when compared with non-diabetic subjects. This combination of increased platelet activity and an impaired response to antiplatelet drugs makes finding optimal antithrombotic regimens for use in people with DM and using them early in the disease before atherothrombotic complications can develop an important goal.
The authors explore trial work looking at low–dose aspirin for primary CVD prevention in DM and suggest options for improving the benefit/risk profile of aspirin in this population such as the use of gastroprotectants e.g. proton pump inhibitors (PPIs).
For further information please see:
Rocca B and Patrono C. Aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: A new perspective. Diabetes research and Clinical Practice 2020; 160: 108008.
Aspirin for primary cardiovascular disease (CVD) prevention in people with diabetes mellitus (DM).
DM increases the rate of first and recurrent CVD events mainly because of the effect DM has on enhancing platelet adhesion, activation and aggregation and because high blood glucose results in an osmotic effect and oxidative stress. In addition, insulin resistance decreases the bodies response to atherosclerosis and results in endothelial dysfunction.
The article reviews data from clinical trials including ASCEND, ARRIVE, ASPREE and JPAD and looks at the delicate risk benefit equation for CVD prevention versus bleeding side effects encountered with low-dose aspirin use. They suggest gastroprotectants as one way to modify this.
The authors state:
“Shared decision-making taking into account the patient’s age, bleeding history and risk, cardiovascular risk factors, quality of life, preferences, and willingness to undergo long-term aspirin therapy; is necessary.“
For further information please see:
Khalil S, Darmoch Fh z and Alraies MC. Should all diabetic patients be on aspirin for primary prevention? Expert review of cardiovascular therapy 2019; 17;8;557-560
Antiplatelet therapies in diabetes – an invited review for Diabetic Medicine
– Diabetes UK
A combination of advanced vascular pathology and an enhanced thrombotic environment result in cardiovascular complication being a major cause of mortality and morbidity for people living with diabetes. The metabolic changes in diabetes that contribute towards this thrombotic environment include poor glycaemic control, insulin resistance and raised cholesterol. Further metabolic changes (enhanced P-selectin expression, increased intracellular calcium, decreased platelet membrane fluidity, reduced nitrous oxide production, altered platelet signally) result in increased platelet activation, adhesion and aggregation.
The authors point out that as well as the personal burden, diabetes consumes around 10% of UK health budget of which a large proportion is spent managing vascular complications. The review aims to give a pragmatic guide for the clinician on the management of thrombotic risk in people with diabetes.
The review covers primary and secondary prevention of CVD for people with diabetes. The authors conclude that whilst low-dose aspirin remains the ‘preferred agent for long-term secondary cardiovascular prevention’ questions remain about its role in primary prevention. Those at higher risk can be considered for primary prevention with low-dose aspirin and these may include people with a longer duration of diabetes, those with significant microvascular disease and smokers providing they do not have a higher bleeding risk.
The increased platelet turnover in diabetes may make more frequent dosing necessary but more research is required in this area to increase our understanding of how this would work in practice.
The authors review the findings from the landmark ASCEND study and whilst the authors of the ASCEND study conclude that the vascular benefits were negated by bleeding side effects the review authors suggest this is not necessarily the case for the following reasons:
- The number needed to treat for a CVD prevention benefit is 91 whilst the number needed to harm is 128.
- Most bleeding events were upper gastrointestinal, and this could have been reduced with the co- prescribing of gastroprotectants.
- The participants in ASCEND had good glycaemic control and it maybe those with poor control would demonstrate a greater benefit
- The dosing schedule may not have been optimal. The dose chosen of 100mg aspirin daily may have been too high for the best risk benefit profile or a twice daily dosing regimen may have been better.
Smokers are identified as a high-risk group and potential issues with cigarette smoking causing increased platelet activation are discussed. Antiplatelet strategies for people who continue to smoke with diabetes need to address these challenges.
The authors call for future research to help identify people more at risk of CVD events and strategies to monitor the response to antiplatelet therapies. They also suggest studies to look at any potential differences between type 1 and type 2 diabetes in terms of CVD prevention.
For further information please see:
Sagar RC, Naseem KM and Ajjan RA. Invited Review. Antiplatelet therapies in diabetes. Diabet. Med. 2020 37,726-734.
Aspirin and primary cardiovascular prevention – decisional strategy based on risk stratification.
The authors of this paper explain that in part the results of three recent aspirin primary cardiovascular disease (CVD) prevention studies (ASCEND, ARRIVE, ASPREE) may have been affected by a progressive reduction in major adverse cardiovascular events (MACE) due to global education programmes that have resulted in people smoking less, exercising more and using lipid lowering therapies to reduce CVD risk.
The authors interpret the results from ARRIVE in the context of the low incidence (4%) of MACE observed in the study and suggest that the negative benefit to risk ratio seen should not be generalised to populations of higher CVD risk.
In the ASCEND trial, where the overall CVD benefits were somewhat negated by the bleeding side effects of aspirin, only 17.2% of the participants had a 5-year CVD risk over 10% making this a low-moderate risk population. In addition, only around a quarter of the participants were given gastroprotectants and its is felt that routine prescribing of a proton pump inhibitor (PPI) would increase the overall clinical benefit from aspirin primary prevention therapy.
The ASPREE trial showed that in healthy elderly people, over 70 (or over 65 if black or Hispanic in the USA) aspirin primary prevention is not likely to be beneficial.
The authors discuss how there is evidence that the net benefit of aspirin increases with the 10- year risk of MACE while the relative risk of having a major bleed remains the same. They suggest considering aspirin for primary CVD prevention where the 10-year risk of MACE is 10-20% and advising aspirin use when this 10-year risk is over 20%. In addition, an individual’s bleeding risk and potential to benefit from other non- vascular benefits of aspirin therapy, such as colorectal cancer prevention, can be considered. The authors have developed a very practical stepwise algorithm to aid clinical decision making around aspirin and primary CVD prevention and within this PPI co-prescribing is routinely considered.
Accurate CVD risk stratification tools, bleeding risk assessment tools and clear decisional algorithms would help enable appropriate individualised prescribing of aspirin for primary CVD prevention. The authors suggest that new CVD risk scores with a greater number of risk factors will be helpful and tools such as artificial intelligence and deep learning may further enhance our decision making. Bleeding risk estimation needs further study but evidence so far suggests that models that include age, ethnicity, socioeconomic deprivation as well as clinical measures such as BP and cholesterol profile , family history of premature CVD, smoking, diabetes, bleeding, peptic ulcer disease, cancer, chronic liver disease, chronic pancreatitis or alcohol-related conditions and medication use such as NSAIDs, corticosteroids and SSRIs may have the potential to aid the prediction of major bleeding events.
The concept of weighted net clinical benefit is also discussed in which MACE and major bleeding events are weighted in terms of their impact on morbidity and mortality. An extracranial (e.g. GI) bleeding event is usually less serious than an MI or ischemic stroke. This is especially pertinent when you consider that many GI bleeding events could be prevented if gastroprotection is employed as part of the overall disease prevention strategy.
The authors state:
“Following general preventative measures (physical exercise, smoking cessation, treatment of hypertension and hypercholesterolemia, etc.), whose benefits are clear and risks remarkably low, an individualised approach to aspirin prescription is warranted. When patients are less than 70 years of age, clinicians should assess the 10-year CV risk. When that risk is very high and the bleeding risk is low, aspirin treatment should be considered after taking into account the patient’s preferences.“
For further information please see:
Aimo A and De Caterina R. Aspirin for primary prevention of cardiovascular disease: Advice for a decisional strategy based on risk stratification. Anatol J cardiol. 2020;23(2):70-78.
Diabetes and coronary artery disease: not just a risk factor.
In this article the authors explore the importance of cardiovascular disease as a complication of diabetes and the importance of looking beyond glycaemic control to manage it. They explore the complex relationships that exist within the ‘metabolic milieu’ that is associated with diabetes and atherosclerotic cardiovascular disease (ASCVD). For instance, the development of renal impairment with uncontrolled diabetes also increases ASCVD risk and there is growing recognition that unrecognised heart failure is increased where both renal and cardiovascular disease exist further adding to the overall disease burden suffered by the individual.
ASCVD is precipitated by multiple risk factors and these need to be considered when estimating a person’s overall CVD risk. Moderate, high and very high ASCVD risk categories can be used in diabetes based on duration of the diabetes, the presence of CVD risk factors and/or target organ damage. Based on this aspirin can be considered for those at high and very high risk of CVD.
The authors call for more collaboration between specialities with diabetologists working closely with cardiologists
The authors state that:
“All prescribers should understand the array of diabetes and non-diabetes drugs available for use in CVD.”
In conclusion the authors reflect that:
“Overall, diabetes therapy has moved towards a more individualised approach based on a wealth of available data.”
For further information please see:
Grant PJ, Cosentino F and Marx N. Diabetes and Coronary artery disease: not just a risk factor. Heart 2020; 0:1-8. Doi:10.1136/heartjnl-2019-316243. Epub ahead of print
The American Diabetes Association 2020 CVD risk management antiplatelet guidance
The American Diabetes Association (ADA) recommends low-dose aspirin (75-162 mg/day) for secondary prevention in people with a history of cardiovascular disease (CVD). For those unable to take aspirin clopidogrel (75mg/day) can be used. Dual antiplatelet therapy with low-dose aspirin and a P2Y12 inhibitor is recommended for at least a year after acute coronary syndrome therapy (ACS).
In the case of primary CVD prevention, the ADA state that;
“Aspirin therapy (75-162mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased cardiovascular risk, after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding.”
The ADA review clinical trial work including the Antithrombotic trialists’ collaboration, ASCEND, ARRIVE and ASPREE and conclude that aspirin appears to have a modest impact on decreasing ischemic vascular events especially where atherosclerotic cardiovascular disease (ASCVD) risk is higher. This however is tempered by its main side effect of increased gastrointestinal (GI) bleeding which may be as high as 5 per 1,000 per year in the real-world setting. Where ASCVD risk is more than 1% per year the number of ASCVD events that are averted are comparable to the number of GI bleeding events caused. The ADA however recognise that CVD events and GI bleeding events ‘do not have equal effects on long-term health’.
The ADA explain that men and women age 50 years or older with diabetes and one additional CVD risk factor such as family history of ASCVD, high blood pressure, high cholesterol, smoking, chronic kidney disease and who are not at increase bleeding risk e.g. older age, anaemia, renal disease, are most likely to benefit from using low-dose aspirin for the primary prevention of ASCVD. They also suggest that non-invasive imagining techniques could potentially help identify people for aspirin primary prevention therapy.
For further information please see:
American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43(suppl.1): S111-S134
The European Society of Cardiology guidelines on diabetes, pre-diabetes and cardiovascular disease recommendations on antiplatelet therapy.
The ESC 2019 guidelines on diabetes (DM), pre-diabe- tes, and cardiovascular disease state:
- Patients with DM and symptomatic CVD should be treated no differently to patients without DM
- In patients with DM at moderate CV risk, aspirin for primary prevention is not recommended
- In patients with DM at high/very high risk, aspirin may be considered in primary prevention.
In addition, the ESC 2019 diabetes guidelines identify the following gaps in the evidence relating to antiplate- let use in people with diabetes:
- Type 1 diabetes and CVD prevention [in vivo plate- let activation has been reported]
- Body mass and antiplatelet responsiveness especial- ly in those with diabetes and obesity where higher dose strategies need investigation
- Are antithrombotic effects similar in pre-DM and DM?
For further information please see:
Cosentino F, Grant PJ, Aboyans V et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, prediabetes, and cardiovascular disease of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). European Heart Journal 41;2: 255-323.
Extracts from the 2019 International Aspirin Foundation Scientific Conference, Rome, 2019: benefits and risks of antithrombotic therapy for cardiovascular disease prevention
Benefits and risks of antithrombotic therapy in primary prevention was one of the many topics discussed during our Scientific Conference, where international experts gathered to discuss and debate ‘ Targeting the right patient population for primary prevention: the case of diabetes mellitus’
Below are the speaker biographies and their extract from the conference report.
- Gemma Vilahur (Cardiovascular Research Center, Barcelona)
Mechanisms of atherothrombosis in diabetes mellitus - Francesco Cosentino (Karolinska Institutet, Stockholm)
Translating clinical trial evidence into treatment recommendations for the use of aspirin in diabetes
Dr Gemma Vilahur

Dr. Gemma Vilahur is a Senior Researcher at the Cardiovascular Program ICCC at the Research Institute of the Hospital de la Santa Creu i Sant Pau (Barcelona) where she coordinates the Translational Research Department. Her previous appointments include: Post-doctoral fellowship from the Spanish Ministry of Economy and Competitiveness at the Cardiovascular Biology Research Laboratory, Zena and Michael A. Wiener Cardiovascular Institute at the Mount Sinai School Medicine, New York, USA (2004-2006); Juan de la Cierva Researcher at the Spanish Ministry of Science and Education, (2006-2009); Ramon y Cajal Researcher from the Science and Innovation Ministry of Spain (MICINN; 2010-2015).
She has published 123 articles (Web of Science; 2.962 citations, H index = 31) and include original manuscripts, consensus and position papers, and reviews. In addition, she has contributed to 29
book chapters.She is and has been principal investigator and co-investigator of 40 research projects (National and European projects either funded by public agencies or industry) and has collaborated in two projects of the 7th Framework EU Program. Besides, she has been involved in a CENIT research program (Spanish Ministry of Science and Innovation; 2010-2015).
Dr. Vilahur’s Awards and Honors include: European Society of Cardiology – ESC [Berlin 2002, Vienna 2003, Vienna 2007, Munich 2008 (young investigation award), London 2015]; first Prize National Congress of Cardiology (Madrid 2007; Seville 2012); first and second Prize in the National Congress
of Atherosclerosis (Pamplona 2009 and Zaragoza 2013, respectively); Prize from the Northwestern Cardiovascular Young Investigator’s Forum; the Arteriosclerosis, Thrombosis, and Vascular Biology Merit Award of the American Heart Association (AHA; Chicago 2006); and award by L’Oreal-UNESCO foundation – for Women in Science (2012).
Mechanisms of atherothrombosis in diabetes mellitus
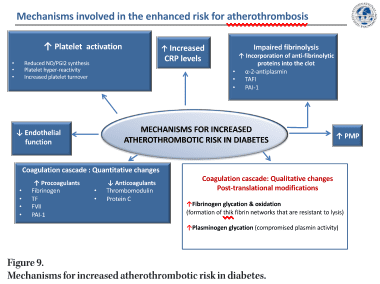
In the final paired session of the day, Dr. Gemma Vilahur (Cardiovascular Program ICCC – Research Institute Hospital Santa Creu i Sant Pau, Barcelona, Spain) explored the mechanisms of atherothrombosis in DM (Figure 9). DM is a global emergency and represents a major cause of cardiovascular morbidity and mortality in developed countries with atherothrombosis being the most common cause of death. It is estimated that 8/10 individuals with DM will die from a cardiovascular event. Multiple pathophysiological mechanisms are thought to account for the pro-atherosclerotic and pro-thrombotic status associated with diabetes, such as obesity, dyslipidemia, and low-grade inflammation.
Diabetic patients display a less thromboresistant vasculature mainly because of impaired endothelial function and a consequent decline in the production of key protective molecules, including nitric oxide (NO) and prostacyclin. In addition, the platelets of diabetic patients have an enhanced turnover, they have over-production of thromboxane and exhibit a hyper-reactivity phenotype derived from a dysregulation at both the receptor and the intracellular signal transduction levels. On top of this, quantitative and qualitative changes of haemostatic factors account for the diabetic hypercoagulable state which, in concurrence with impaired endogenous fibrinolysis, leads to denser clot structures and delayed spontaneous clot lysis.
Preliminary studies using intracoronary angioscopy have demonstrated that patients with T2DM not only had an increased incidence of ruptured atherosclerotic plaques in their coronary circulation, but there was virtually a doubling of intravascular thrombi in these patients, suggesting an abnormal tendency towards thrombus formation or clot dissolution [64].
The endothelium, a single layer of cells in the interior of blood vessels, provides a physical barrier. Its main purpose is to maintain vascular homeostasis regulate vascular tone and smooth muscle cell proliferation, reduce inflammation and prevent thrombosis [65]. Endothelial dysfunction can thus be used as a critical surrogate endpoint for CVD [66–68]. Insulin resistance and sustained hyperglycemic levels experienced with DM directly affect endothelial activity, inhibiting insulin-mediated protective mechanisms, and stimulating maladaptive responses. In its normal protective and physiological state, insulin interacts with insulin receptors leading to the synthesis of two important vasodilators and antithrombotic agents, NO and prostacyclin (PGI2) [69–71]. Insulin resistance with DM diminishes these key protective mechanisms resulting in reduced vasodilation and the loss of antiplatelet effects. In addition, hyperglycaemia leads to an increase in systemic oxidative stress (reactive oxygen species (ROS) production) [72]. This enhanced ROS production compromises NO synthesis and stimulates endothelial inflammation via several cellular mechanisms, including promoting activation of PKC and NF-κB signalling. NF-κB, in turn, induces the transcription of inflammatory response-associated genes [73] and vascular adhesion molecules further stimulating leukocyte recruitment, thereby aggravating the inflammatory process [74]. ROS also reduces NO levels by eNOS downregulation and induces the formation of highly oxidant peroxynitrite ion and asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of eNOS activity. Diabetic patients not only produce an excess of ROS but their antioxidant mechanisms are also impaired (e.g. reduced superoxide dismutase) [73, 75]. The prolonged oxidative response that occurs during hyperglycaemic states results in the formation of advanced glycation end products (AGEs) by glycosidation of proteins and fatty acids [74]. AGE interaction with its receptors (RAGE) maintains endothelial dysfunction by promoting the release of pro-inflammatory cytokines and cell adhesion molecules, compromising the endothelial barrier function and leading to increased vascular leukocyte infiltration. Finally, recent data have suggested that red blood cells also contribute to DM-related endothelial dysfunction by increasing endothelial arginase-1 activity, an eNOS competitor for L-arginine substrate [76, 77].
Multiple factors are involved in the increased platelet reactivity observed in DM patients [78]. Hyperglycaemia increases Ca2 mobilisation from intraplatelet storage pools leading to an increased intracellular Ca2 level and the consequently enhanced sensitivity to aggregating agents. People with DM have impaired insulin receptors [71] and the altered signalling of these platelet insulin receptors promotes the expression of adhesion molecules (GpIIb/ IIIa, P-selectin) and prothrombotic agonists (thrombin, ADP, TxA2). Platelets from people with DM synthesise more TxA2 than normal platelets in response to a variety of agonists that induce deacylation of arachidonate from membrane phospholipids and show hyper-responsiveness of proteinase-activated receptor (PAR) to thrombin and of the P2Y12 receptor to ADP [71, 78]. In addition, DM patients have an enhanced platelet turnover and this increases the proportion of highly reactive, newly formed platelets with lower platelet fluidity due to changes in membrane lipid structure or glycation of membrane proteins and with alteration in its intracellular components [79].
Dr. Vilahur described an experimental animal model in which crossed-bone marrow transplants were performed between Zucker lean and Zucker Fatty/insulin-resistant rats. This study showed that alterations in platelets produced by diabetic bone marrow megakaryocytes contribute to the enhanced thrombotic risk observed in DM [80]. Interestingly, the presence of insulin resistance was already capable of modulating bone marrow released platelets enhancing their susceptibility to form thrombi [81–82]. The work also proved that the bone marrow from diabetic donors induces pro-atherogenic modifications in healthy recipients, increasing their risk of developing atherosclerosis lesions [83].
The low-grade inflammation and oxidative stress seen in DM patients contribute to platelet reactivity through endothelial dysfunction (reduction in eNOS activity) and increased lipid peroxidation to generate F2-isoprostanes which are thought to amplify platelet activation by low concentrations of other agonists. The low-grade inflammatory state triggers IL-6, fibrinogen, and C-reactive protein (CRP) secretion. Elevated CRP levels constitute an independent risk factor associated with increased cardiovascular mortality in DM [84]. CRP has been shown to enhance the expression of endothelial adhesion molecules, stimulate macrophages
to synthesise cytokines, and induce TF expression in monocytes. Additionally, CRP has been reported to modify the fibrinolytic balance of endothelial cells and thus promote fibrin formation, to enhance the expression of PAI-1 in human endothelial cells and inhibit tPA activity. A modified form (monomeric form) of CRP has the ability to contribute to thrombus progression and growth [85–86]. Platelet-derived microparticles (small membrane vesicles released from the surface or plasma membrane of cells upon activation or death) are found at higher levels in people with DM [87] and can exert both pro-inflammatory and pro-thrombotic effects which may contribute to the atherothrombotic response [88–89].
As well as reduced endothelial thrombo-resistance and enhanced platelet activation people with DM often have hypercoagulable blood [90]. Hyperglycaemic states can exert direct effects on the gene transcription of coagulation factors [91] and DM is associated with increased plasma levels and activity of various coagulation factors (TF, factor VII, TF–coagulation factor VIIa complex activity, and factor XII) resulting in enhanced thrombin production. Also, plasma levels of fibrinogen (the soluble precursor of solid fibrin) are increased in diabetes, as part of the ongoing low-grade inflammation. In counterpart, natural anticoagulants such as thrombomodulin, protein C and antithrombin III are found to be reduced in diabetes further developing the prothrombotic environment. Altogether, these changes culminate in increased thrombin generation and fibrin network formation, which is characterised by increased density and improved resistance to fibrinolysis. DM also contributes to thrombotic complications by altering the fibrinolytic mechanisms. As such, diabetic patients have decreased tissue plasminogen activator (t-PA) and enhanced anti-fibrinolytic activity explained through an increase in plasminogen activator inhibitor-1 (PAI-1) and carboxypeptidase B2 (also known as thrombin-activable fibrinolysis inhibitor), thus hampering the conversion of plasminogen to plasmin [92]. Increased levels of PAI-1 are mostly associated with insulin resistance since they are predominantly observed in T2DM patients, but not in other hyperglycaemic situations. Hyperglycaemia can also directly affect the fibrinolytic system by increasing plasminogen glycation (posttranslational modification), thereby adversely affecting its conversion to plasmin [93].
In conclusion, DM is a chronic metabolic disorder in which a hyperglycaemic state and insulin resistance promote a low-grade inflammatory background and systemic oxidative stress which leads to accelerated atherosclerotic progression and an increased risk of thrombosis. Other co-morbidities associated with DM such as obesity, hypertension, and dyslipidaemia further contribute to atherothrombotic complications. The reasons for this adverse cardiovascular profile in people with DM are due to several molecular and cellular pathways that combine to enhance atherosclerosis progression and thrombus formation.
Professor Francesco Consentino
 Prof. Francesco Cosentino obtained his MD degree in 1987 and specialty training in Internal Medicine and Cardiovascular Disease at the University of Rome. In 1991 moved to Mayo Clinic & Foundation, Rochester, MN, USA for a Cardiovascular Fellowship. During his stay at Mayo he fulfilled all the requirements for a PhD in Biomedical Sciences – Cardiovascular Pharmacology. In 1995, he joined the Cardiovascular Division at the University Hospital of Bern. Two years later, Francesco Cosentino moved to the Division of Cardiology of Zurich University Hospital as “lecturer” and then “titular professor” of Cardiology. In 2006, he was appointed associate professor of Cardiology at the University of Rome “Sapienza”.
Prof. Francesco Cosentino obtained his MD degree in 1987 and specialty training in Internal Medicine and Cardiovascular Disease at the University of Rome. In 1991 moved to Mayo Clinic & Foundation, Rochester, MN, USA for a Cardiovascular Fellowship. During his stay at Mayo he fulfilled all the requirements for a PhD in Biomedical Sciences – Cardiovascular Pharmacology. In 1995, he joined the Cardiovascular Division at the University Hospital of Bern. Two years later, Francesco Cosentino moved to the Division of Cardiology of Zurich University Hospital as “lecturer” and then “titular professor” of Cardiology. In 2006, he was appointed associate professor of Cardiology at the University of Rome “Sapienza”.
Since 2013 he is professor and chair of Cardiovascular Medicine, Karolinska University Hospital, Solna and Karolinska Institute in Stockholm.
Prof. Cosentino is the recipient of grants and prizes from national and international institutions, research councils and private foundations. He is the leading author of more than 150 original articles published in top-ranking, peer-reviewed journals.
Prof. Cosentino is past secretary-treasurer of the European Society of Cardiology and current chair of ESC Partnership and Policy Committee. He was ESC chairman of 2019 ESC/EASD Guidelines on diabetes, pre-diabetes and cardiovascular disease and Associate Editor of European Heart Journal.
Translating clinical trial evidence into treatment recommendations for the use of aspirin in diabetes
Prof. Francesco Cosentino (Karolinska Institutet, Stockholm) gave the final talk of the day providing a very logical journey through primary prevention trials and aspirin in which he looked at the translation of clinical trial evidence into treatment recommendations for aspirin use in diabetes management (Figure 10).
By reducing platelet activity aspirin reduces atherothrombotic events but increases the risk of bleeding events such as GI and intracranial bleeding. In a cohort study of 1.9 million people, 17.9% of people with DMT2 experienced a cardiovascular event over a 5.5-year period [94].
Primary prevention with aspirin is controversial due to the risk-benefit debate. This is of particular concern where the risk of a cardiovascular event is low. There is a high degree of heterogeneity within the trials designed to study the impact of aspirin for primary prevention in general and this has resulted in heterogenicity within the results.
The ASCEND trial [95] showed that, while aspirin did prevent vascular events, it also caused major bleedings. Within ASCEND, one in four of the patients took a PPI and increasing this ratio may reduce bleeding complications.
Prof. Cosentino discussed the issues around whether a cardiovascular event is a worse event than a major bleed. With the exception of intracranial bleeding as long as you do not die of a GI bleed on the whole the experience probably
has less impact on a person than having a CVD event. The other complication to using aspirin for primary prevention is that a person’s risk is not static but changes over time; for example, if they adopt a healthier lifestyle their CVD risk reduces. Starting medication to reduce BP or cholesterol will also reduce a person’s overall CVD risk thus impacting the primary prevention risk benefit ratio for aspirin use. Risk is not static.
What has been learnt?
- ‘It is not enough to simply count up cardiovascular events prevented versus bleeding events (most of which are GI) that have been caused as they do not have the same immediate or eventual health effect.’
- ‘Cost-effectiveness analyses can be helpful provided that we agree with the assumptions used to build the models’
- ‘The greatest potential net benefit occurs in patients with increased cardiovascular risk who are not at increased risk for bleedings (50–59-year-old subjects with a 10-year cardiovascular risk over 10%)’
- Appropriate decision model estimating the ischaemic versus bleeding risk threshold is lacking.’ ‘Perhaps new genetic markers and risk estimators derived from artificial intelligence (AI) may help to refine risk assessment.’
The 2016 European guidelines [96] on CVD prevention in clinical practice with a class 3, level B rating do not recommend antiplatelet therapy for individuals without CVD due to the increased risk of a major bleed.

As of 31 August 2019 a third set of collaborative guidelines produced jointly by the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) –
2019 ESC Guidelines on diabetes, pre-diabetes and cardiovascular diseases developed in collaboration with the EASD.
https://academic.oup.com/eurheartj/article/41/2/255/5556890
These guidelines explain that patients with DM and symptomatic CVD should be treated the same as people without DM when it comes to antiplatelet therapy.
Low-dose aspirin [75-100 mg per day] maybe considered for people with diabetes mellitus [DM] who also have a high risk of cardiovascular disease [CVD] and no clear contraindications to aspirin.
For a summary:
https://www.aspirin-foundation.com/scientific-information/disease-prevention/diabetes-guidelines/