Chemistry
The Chemistry of Aspirin (acetylsalicylic acid)
Chemical formula = C9H8O4 or CH3COOC6H4COOH or HC9H7O4
Aspirin is prepared by chemical synthesis from salicylic acid, through acetylation with acetic anhydride. The molecular weight of aspirin is 180.16g/mol. It is odourless, colourless to white crystals or crystalline powder.
Aspirin is an oral non-steroidal anti-inflammatory drug (NSAID) that is rapidly absorbed from the stomach and the small intestine. It is a non-selective NSAID as it irreversibly inhibits both cyclooxygenase (COX) enzymes involved in converting arachidonic acid to prostaglandins and thromboxane3.
Prostaglandins are found throughout the body and are made to help manage injury or infection. Prostaglandins upregulate the sensitivity of pain receptors. As a control mechanism, they act locally at the site of synthesis which limits the extent of their activity. They are also broken down rapidly by the body. The enzymes that produce prostaglandins are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), they have diverse roles and are widely dispersed throughout body tissue. Cox-1 has a protective role for the stomach lining and COX-2 is involved in pain and inflammation. Aspirin binds to and acetylates serine (an amino acid used by the body to make proteins) residues in the active site of cyclooxygenase enzymes, leading to reduced production of prostaglandin. This in turn mediates aspirin’s effect of reduced inflammation and pain in affected tissues. Additionally, aspirin acts on prostaglandins in the hypothalamus to reset and reduce a raised body temperature. Importantly, aspirin does not decrease normal body temperature1,2,3.
From a cardiovascular perspective aspirin also has an important role: Thromboxane A2 (TXA2) is a lipid that stimulates new platelet formation and increases platelet aggregation. Aspirin inhibits the production of thromboxane A2 (TXA2) by stopping the conversion of arachidonic acid to TXA2. This aspirin effect is mediated via COX-1 inhibition within platelets and helps stop the platelets from sticking to each other or to plaques within the artery therefore reducing the risk of blood clot (thrombus) formation within the blood stream. In this way aspirin can help lower the risk of future myocardial infarction (MI) or stroke1,3.
Aspirin, therefore, has an analgesic (reduces pain), anti-inflammatory (reduces redness and swelling), anti-platelet (reduces blood clots) and antipyretic (temperature reduction) effects1,2,3.
In cancer, aspirin is believed to impact a number of cancer signalling pathways and may induce or upregulate cancer suppressor genes3.
Because Aspirin is a non- selective COX- 1 and COX-2 inhibitor, as well as its beneficial analgesic, anti-inflammatory, anti-platelet and antipyretic effects its use can also result in peptic ulcer development and gastric bleeding. Taking aspirin and alcohol together can increase the risk of gastric bleeding 1,3.
Inside the body, aspirin is converted into its active metabolite salicylate. This happens mostly in the liver. Peak concentration of salicylate in the plasma occurs approximately 1-2 hours after ingestion. Excretion from the body is mainly through the kidney. Alkaline urine speeds up the excretion of aspirin. It takes about 48 hours to excrete an aspirin completely. The half-life of aspirin in the blood stream is 13-19 minutes and the half-life of its metabolite salicylate is around 3.5-4.5 hours. Aspirin’s inhibition of COX-1 results in reduced platelet aggregation for the 7-10-day average lifespan of platelets1.
There is a 60% structural similarity between COX-1 and COX-2 active sites: The active site of COX-2 is larger and this allows the precursor of prostaglandins, arachidonic acid, to be able to bypass aspirin molecules at lower doses. Therefore, a higher dose of aspirin is required for its analgesic and anti-inflammatory effects in comparison to its antiplatelet action1. The fact that COX-1 and COX-2 enzymes have different levels of sensitivity to aspirin and recover their cyclooxygenase activity post aspirin at different rates helps explain the different dosing regimens for aspirins varying clinical indications1.
Aspirin should not be used in children as it can produce a rare but dangerous Reye’s syndrome resulting in coma and liver damage that can prove fatal1,3.
Some drug interactions can occur when aspirin is given with other medicines. Aspirin can displace drugs from their plasma binding-sites and in this way may increases the effects of anticoagulant drugs and oral hypoglycaemics. It can also inhibit urate secretion and should be avoided in gout3.
Raw Materials
Phenol C6H5-OH
Sodium Hydroxide NaOH
Carbon Dioxide CO2
Acetic Anhydride CH3COOCOCH3
Hydrogen H
The Reactions
The production of aspirin from raw materials can be divided into four separate reactions as shown here:
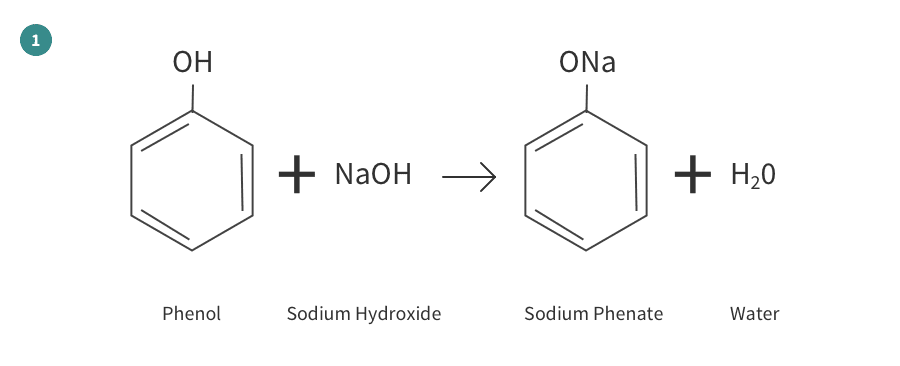
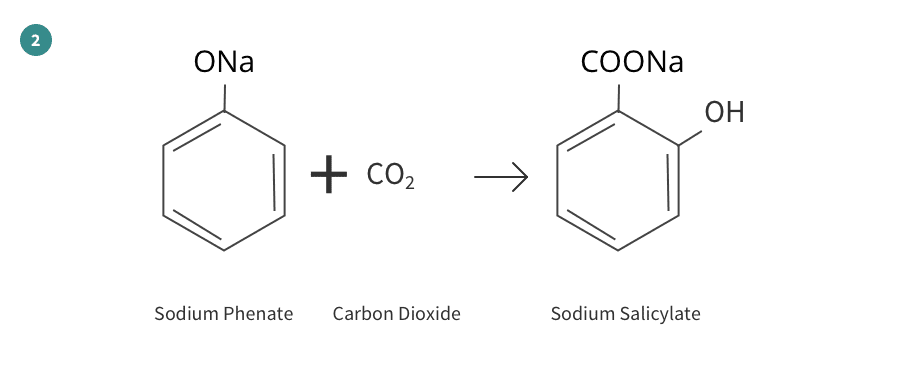
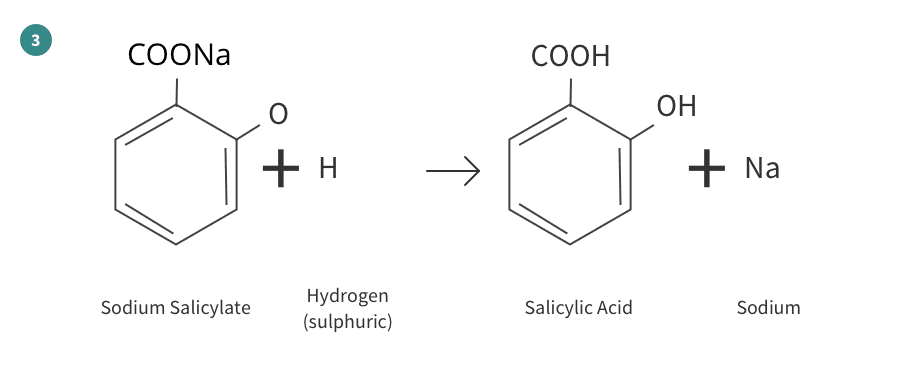
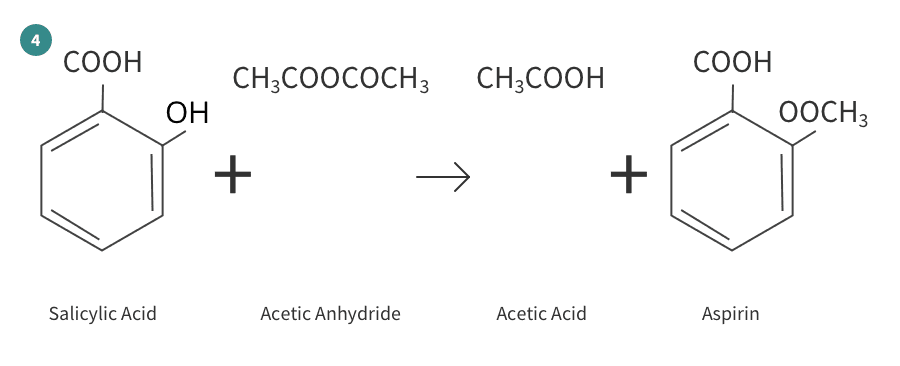
References/Biography
1. PubChem Aspirin compound summary accessed 07/05/2020 https://pubchem.ncbi.nlm.nih.gov/compound/Aspirin
2.You and Your Hormones Prostaglandins accessed 07/05/2020 @
https://www.yourhormones.info/hormones/prostaglandins
3.Trounce’s Clinical Pharmacology for Nurses. Chapter 11 Anti-inflammatory drugs: treatment of arthritis and gout. The non-steroidal anti-inflammatory drugs.











