Aspirin dose and dosing regimen
Introduction
There is increasing interest in the correct dosing regimens for aspirin in different situations. Body weight and disease states may impact on the dose required to maximise beneficial effect. Understanding more about the pharmacology of aspirin and the impact different factors can have on this can help scientists and clinicians devise the most appropriate individualised dose in different scenarios.
The effect of aspirin according to dose, body weight and height on the risk of vascular events and cancer
This study, from 2018, analysed ten randomised trials of aspirin in the primary prevention of disease involving 117,279 participants and found that body size had an important impact. Different weight bands for bodyweight (10kg bands) and height (10cm bands) were analysed to compare the effects of low-dose aspirin (<100 mg) and higher doses of aspirin (>300mg). The findings were stratified according to age, sex and vascular risk factors.
The researchers found that the best dose of aspirin to prevent cardiovascular events is dependent upon bodyweight and that lean body mass and height are more important factors than body-mass index. They discovered that the commonly used once-daily low- doses of 75-100mg of aspirin were not effective in people weighing in excess of 70kg especially where enteric coated formulation were used or where the person also smoked. The impact of aspirin in preventing sudden cardiac death and cancer was also influenced by dosing and weight. This study confirmed that aspirin did reduce in-trial death from cancer after 5 years of follow up but interestingly only in people weighing less than 70kg.
The authors suggest that the loss of effect of low-dose aspirin at larger body size, which is driven more by weight and height than BMI, is more likely to be due to insufficient systemic bioavailability of aspirin rather than an increased platelet activation caused by obesity.
The authors conclude:
“the one- dose- fits- all strategy for daily aspirin use is unlikely to be optimal. The substantial reductions in cardiovascular events and death at optimal doses for weight highlight the potential to improve effectiveness and argue for a more tailored dosing strategy.”
For further information please see:
Rothwell PR., Cook N., Gaziano JM. Et al. Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet (2018) 392:387-99
Very-low-dose twice-daily aspirin as part of dual anti-platelet therapy for acute coronary syndrome (ACS)
This study examines the pharmacodynamics of dual antiplatelet therapy using very-low-dose aspirin taken twice-daily with standard-dose ticagrelor. Two dosing regimens for aspirin were compared 75mg once-daily versus 20 mg every 12 hours to see if the lower but more frequent dose could maintain antithrombotic benefits whilst reducing bleeding risk.
The study had a randomised cross-over design and assessed 20 ticagrelor-treated ACS patients on the differing aspirin dosing regimens for 14 days before swapping to the other regimen.
After 14 days of therapy the group taking the twice-daily 20 mg aspirin dose with standard dose ticagrelor had consistent inhibition of platelets and improved post- dose haemostasis.
The authors conclude:
“aspirin dose modification represents a novel and feasible strategy to be investigated for optimizing the balance of antithrombotic benefits and bleeding related risks in ticagrelor-treated ACS patients, and demands further study to determine whether this translates into improvements in net clinical outcomes.”
For further information please see:
Parker W.A.E., Orme R.C., Hanson J., Stokes H.M., Bridge C.M., Shaw P.A., Sumaya W., Thorneycroft K., Petrucci
G., Porro B., Judge H.M., Ajjan R.A., Rocca B. and Storey R.F. Very-low-dose twice-daily aspirin maintains platelet inhibition and improves haemostasis during dual- anti-platelet therapy for acute coronary syndrome. Platelets (2019) 30;
Aspirin in the primary prevention of cardiovascular disease for people with diabetes mellitus
In this review article Professors Rocca and Patrono explore the unmet clinical need to further reduce cardiovascular events for people with both type 1 and type 2 diabetes mellitus (DM). Despite progress in improving the control of hyperglycaemia and other cardiovascular risk factors the burden of morbidity and mortality from atherosclerotic cardiovascular disease (ASCVD) for those with DM remains approximately 2-fold higher than those without DM. The article explores the role of low-dose aspirin along with the use of other CVD prevention drugs such as statins, physical activity and diet in tackling and reducing ASCVD risk.
The ‘accelerated atherosclerosis’ seen in people with DM appears to be caused by multiple changes in the body. These include; endothelial dysfunction, platelet activation, low grade inflammation and oxidative stress. The targeting of multiple pathways may be beneficial to help curtail this accelerated development of atherosclerotic changes and the accompanying thrombotic complications.
The authors discuss the ASCEND trial which was powered to show the safety and efficacy of low- dose aspirin use to reduce vascular events in people with DM. The effect of aspirin in reducing cardiovascular events is additive to the benefit gained from statins and drugs to control blood pressure. This benefit from aspirin is also independent of the underlying CVD risk. The risk versus benefit profile of low-dose aspirin use can be improved using gastroprotectants. Future individualised dosing strategies for aspirin may further help our understanding of its role in reducing first CVD events in people with DM. The ANDAMAN trial has been set up to explore individualised dosing regimens for the growing number of obese patients with DM to see if twice-daily dose is better than once-daily.
For further information please see:
Rocca B. and Patrono C. Aspirin in the primary prevention of cardiovascular disease in diabetes mellitus: A new perspective. Diabetes research and clinical practice (2020) 160 108008.
Obesity is associated with reduced response to once-daily low-dose aspirin
Current antiplatelet drug regimens have, for the most part, been designed and tested in a nonobese population. Obese people are at increased risk of cardiovascular disease making adequate dosing strategies of utmost importance.
This pilot study explores the pharmacodynamics of once-daily low-dose aspirin in 16 healthy aspirin naïve obese people in order to understand how body weight and body mass may impact on aspirin pharmacology. Age and sex-matched non-obese controls were included for comparison. The study found that rising body size was linked to impaired aspirin pharmacodynamics and high platelet activation.
An in-silico model was used to predict the aspirin dose required for these subjects and found that a dose of 200 mg once-daily or 85mg twice-daily would be needed for an adequate response.
The study indicates that this impaired inhibition of platelet activation by a standard low-dose aspirin regimen may impact on its antithrombotic effect.
The authors conclude:
“a standard, once-daily low-dose aspirin regimen appears to be insufficient to adequately inhibit COX-1 dependent platelet activation in moderately to severely obese subjects.”
For further information please see:
Petrucci G., Zaccardi F., Giaretta A., Cavalca V., Capristo E., Cardillo C., Pitocco D., Porro B., Schinzari F., Toffolo G., Tremoli E. and Rocca B. Obesity is associated with impaired responsiveness to once-daily low-dose aspirin and in vivo platelet activation. J Thromb Haemost. (2019):17:885-895.
Measurement of thromboxane biosynthesis in health and disease
In this article the authors review the research from four decades of work on thromboxane. Thromboxane is a lipid mediator that is involved in several body and disease processes including the formation of a platelet plug (primary haemostasis), atherothrombosis, inflammation and cancer. The article focuses on the measurement of thromboxane A2 (TXA2) metabolites to gain greater understanding of the pathophysiological role of increased platelet activation (transient or persistent) and looks at the clinical pharmacology of COX-1 inhibition.
Platelet TXA2 biosynthesis can be assessed ex vivo by measuring serum TXB2 or in vivo by measuring urinary enzyme metabolites as indexes of platelet activation. The impact of aspirin on platelet COX-1 activity has been studied using serum TXB2 and urinary metabolites of TXB2.
The article helps us to understand the role of body size and aspirin dose. There is biochemical evidence that obesity is linked with greater platelet activation and a higher risk of cardiovascular disease. Standard once- daily low-dose aspirin therapy is therefore inadequate for people with a larger body size and instead an increased dose or a twice-daily dosing schedule is suggested.
The authors conclude:
“Altogether, early and newer evidences have shown the desirability and practicality of adjusting the aspirin dosing interval based on two measurements of serum TXB2 ,at 12 and 24 h after dosing (Pascale et al ., 2012; Rocca et al., 2012), a personalized approach that may be required under conditions of reduced systemic bioavailability of aspirin (Petrucci et al., 2019) or enhanced platelet turnover (Patrono et al 2013). ”
For further information please see:
Patrono C and Rocca B. Measurement of Thromboxane Biosynthesis in Health and Disease. Front in Pharmacology October 2019, volume 10 article 1244 doi: 10:3389.
Increased von Willebrand factor levels in polycythemia vera and phenotypic differences with essential thrombocythemia.
This study seeks to explore the von Willebrand factor (VWF) pattern in polycythemia vera (PV) (48 subjects) and compare this with matched healthy subjects (48) and essential thrombocythemia (ET) patients (41). The researchers found VWF and factor VIII (FVIII) antigen levels in people with PV were higher than in controls and could be predicted by measuring JAK2-p.V617F burden and erythrocyte count.
VWF activity in patients with PV were found to be significantly higher than in matched patients with essential thrombocythemia (ET). In contrast to ET, acquired VWF deficiency was not seen in people with PV. It is thought that the high VWF and factor VIII levels seen in PV maybe implicated in the development of thrombosis. As a result of this, monotherapy with low-dose aspirin may be insufficient to manage the risk of thrombosis and a dual therapy of low-dose aspirin in combination with rivaroxaban may be a more effective regimen.
For further information please see:
Sacco M., Ranalli P., Lancellotti S., Petrucci G., Dragani A., Rocca B. and De Cristofaro R. Increased von Willebrand factor levels in polycythemia vera and phenotypic differences with essential thrombocythemia. Res Pract Thromb Haemost 2020; 4: 413-421.
A randomised, double-blind trial of three aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia.
People with essential thrombocythemia (ET) have abnormal megakaryopoiesis (the process by which platelets develop) and as a result an increased risk of thrombosis. This multicentre randomised, double-blind trial found people with ET treated with a once-daily, low-dose aspirin had insufficient platelet inhibition and that a twice-daily aspirin regimen improved platelet inhibition with no further benefit seen with three times a day dosing.
In this dose-finding part of the ARES (Aspirin Regimens in Essential Thrombocythemia) trial 245 participants with ET were recruited and randomised to receive either 100 mg of aspirin once-daily, twice-daily or three times daily for two weeks. Serum thromboxane B2 (sTXB2) (a biomarker of platelet COX-1 activity) and urine prostacyclin metabolite (PGIM) excretion measured at randomisation and again at 2 weeks were used to assess efficacy and safety. Urinary TX metabolite (TXM) excretion, gastrointestinal tolerability and symptoms related to ET were also studied.
Participants in the twice-daily and three times a day regimen had substantially reduced inter-individual variability and lower median sTXB2 values. Urinary PGIM measures were similar in all three arms of the study and urinary TXM was reduced by 35% in both extra dosing experimental arms. Patients on three times a day aspirin had a higher abdominal discomfort score.
The authors conclude that the current once-daily dosing for the primary or secondary prevention of cardiovascular disease is inadequate for most ET patients and that this can be rectified by giving a twice-daily dose. The ongoing phase of the ARES trial will assess the long-term superiority, tolerability and compliance with twice-daily aspirin dosing in people with ET.
For further information please see:
Rocca B., Tosetto A., Betti S. et al. A randomized, double-blind trial of three aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood 2020 doi:10.1182/blood.2019004596.
Extracts from the 2019 International Aspirin Foundation Scientific Conference, Rome, 2019: benefits and risks of antithrombotic therapy for cardiovascular disease prevention
Benefits and risks of antithrombotic therapy in primary prevention was one of the many topics discussed during our Scientific Conference, where international experts gathered to discuss and debate ‘Optimizing the aspirin dose and dosing regimen’.
Below are the speaker biographies and their extract from the conference report.
- Bianca Rocca (Catholic University, Rome)
Interindividual variability in the extent and duration of platelet thromboxane inhibition by low-dose aspirin - Peter Rothwell (University of Oxford)
Clinical trials of different aspirin doses and dosing regimens
Professor Bianca Rocca
Professor of Pharmacology at the Catholic University School of Medicine in Rome, Italy.

Professor Bianca Rocca, MD, PhD is Associate Professor of Pharmacology at the Catholic University School of Medicine in Rome (Italy).
Professor Bianca Rocca trained as Postdoctoral Fellow at the Center for Experimental Therapeutics, University Pennsylvania of Philadelphia (USA) with Prof. Garret A. FitzGerald. She is immediate Past Chairperson of the European Society of Cardiology (ESC) Working Group on Thrombosis (2018-2020) and ex officio Member of the board of the ESC Working Group on Aorta and Peripheral Vascular Disorders. She is member of the Board of Clinical Pharmacology of the Italian Society of Pharmacology and of the Board of the Italian Group of Atherosclerosis, Thrombosis and Vascular Biology.
She has been part of several Task Forces of guidelines and position papers within the ESC. She has co-authored over 110 articles in peer-reviewed journals with over 6500 citations, including Nature Medicine, Science, Blood, Circulation, Journal of Clinical Investigation, PNAS (USA), Annals of Internal Medicine, ATVB, Nature Clinical Practice in Cardiovascular Medicine, JACC, European Heart Journal, Diabetes. Her H-index is 41. Main scientific topics of interest are antithrombotic drugs, eicosanoids, primary haemostasis, platelets, non-steroidal anti-inflammatory drugs, cardiovascular diseases.
Interindividual variability in the extent and duration of platelet thromboxane inhibition by low-dose aspirin
Prof. Bianca Rocca (Catholic University, Rome, Italy) explained inter-individual variability in the extent and duration of platelet thromboxane inhibition by low-dose aspirin. Understanding interindividual variability of drug responsiveness is important for improving drug effectiveness and safety in the clinical setting and the key objective for precision medicine. Finding ways to better understand what determines variation in aspirin response is important for finding new ways to administer an ‘old’ yet effective, cheap drug in the optimal way.
In a world where patients increasingly have co-morbidities and polypharmacy the fact that aspirin is not metabolised via the CYP450 system is advantageous as this means there is little impact from drug–drug interactions at a pharmacokinetic level on drug responsiveness. Patient characteristics, however, including other diseases can alter the responsiveness to aspirin. In its pre-systemic availability aspirin, before it passes the liver, inhibits circulating platelets by irreversibly blocking the COX-1 enzyme. Based on systemic bioavailability, aspirin acts to inhibit the precursors of platelets in the bone marrow such as megakaryocytes, pro and pre platelets (Figure 7).
Chronic conditions including diabetes, obesity or myeloproliferative neoplasms can influence the extent and/
or duration of platelet COX-1 inhibition and TXA2 generation through disease-specific pharmacokinetic or pharmacodynamics mechanisms. A twice daily low-dose dose regimen restores adequate platelet COX-1 inhibition without effecting vasculo-protective endothelial prostacyclin in conditions with chronically enhanced platelet generation. Serum TXB2ex vivo can act as a pharmacodynamic biomarker surrogate of aspirin efficacy and is used for the approval of new aspirin formulations.
Figure 7.
The pharmacokinetics and pharmacodynamics of aspirin in the human body.
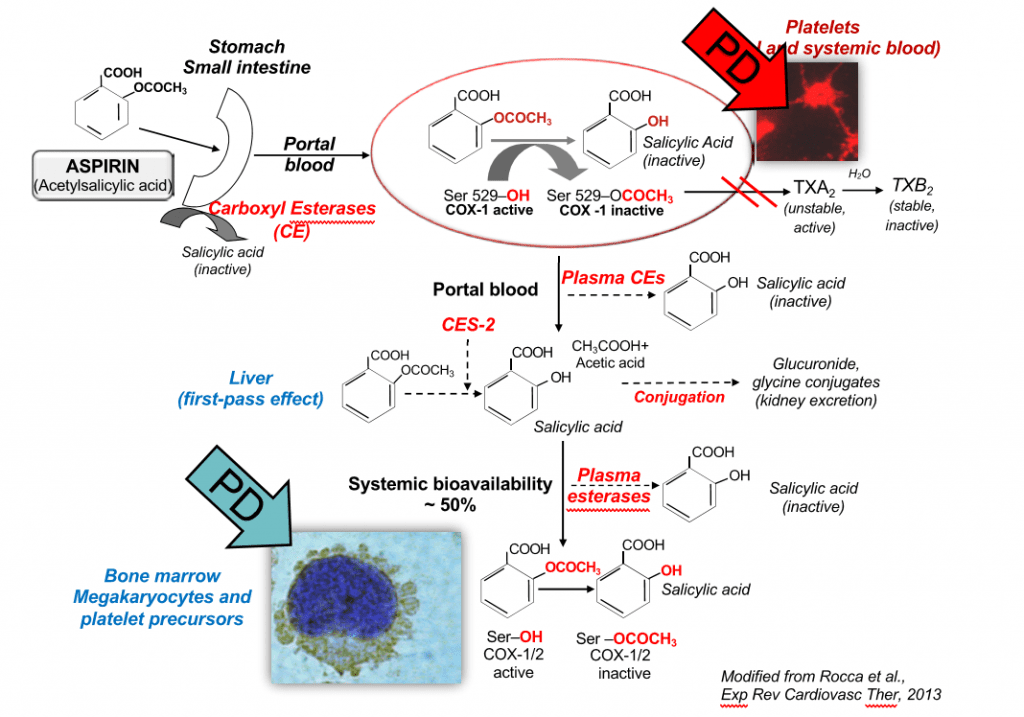
It is hoped that an in silico model of low-dose aspirin pharmacokinetics and pharmacodynamics will help the design of personalised regimens, the so-called ‘precision dosing’, that can then be tested in adequately sized clinical trials of improved versus conventional antiplatelet regimens. This will help build information around how to dose aspirin in patients with moderate-to-severe obesity and/or rare diseases such as essential thrombocythaemia (ET), which are infrequent in or even excluded from traditional randomised controlled trials. It will also help guide aspirin dosing in complex clinical scenarios such as patients with hepatic or kidney impairment, pregnant women, elderly and frail subjects, with different CVDs. The model has an ability to learn when challenged with different real-world data and can be built so that it will be able to advice on aspirin dosing in different individuals, combining different clinical conditions and antropometric features in the future.
Myeloproliferative neoplasms such as ET (where changes within the megakaryocytes lead to an increase in thrombotic events) and Polycythemia Vera (a blood cancer in which the bone marrow makes too many red blood cells causing the blood to become too thick) are associated with a 3.5-fold increase in serious vascular events even with standard low-dose aspirin [49]. Prof. Rocca explained how newly released platelets with unacetylated COX- isozymes released over the traditional dosing interval of once in 24 hours may contribute to the variable lower aspirin responsiveness in ET and that more frequent dosing may be needed. The aspirin regimens in essential thrombocythaemia: (ARES) phase II trial has been set up to test more frequent dosing strategies for aspirin in ET. Initially, twice daily and three times a day regimens will be explored with the best regimen taken forward to the second part of the study, when serum TXB2 measures will be taken every 3 months in order to assess the long-term persistence of superior biochemical efficacy and safety of the optimised dosing regimen.
Currently, we rely on consensus expert advice to guide these clinical scenarios, for example, the ESC Working Group on Thrombosis consensus statement on aspirin and obesity, which advices that while limited data is available on aspirin dosing in people with a body mass index (BMI) above 40 kg/m2, it is reasonable to double the daily low dose of aspirin or give a twice-daily low dose. Recent work has shown a higher residual TXB2 in moderate-to-severe obese people in contrast to non-obese subjects in their response to once-daily low dose aspirin, and in fact, increasing residual serum TXB2 was found to be exponentially correlated with BMI and body weight in 100 subjects taking standard low-dose aspirin (100 mg once daily) [50].
Prof. Rocca concluded that this is just the beginning of the story and that a lot more research and development will be required. Aspirin resistance does not exist; it is low compliance and inter-individual sources of variability that affect the extent of drug response. At variance with drug resistance, which implies the loss of drug effectiveness even by increasing dose, variability can be corrected in the context of precision dosing and medicine.
Professor Peter M Rothwell
Head of the Centre for the Prevention of Stroke and Dementia and Professor of Clinical Neurology, Oxford, UK
 Professor Peter Rothwell qualified in medicine from the University of Edinburgh in 1987 and after completing his early postgraduate clinical training he moved to Oxford as Clinical Lecturer in Neurology in 1996. Professor Peter Rothwell was awarded an MRC Senior Clinical Fellowship in 1999 and set up the Stroke Prevention Research Unit in 2000, which now employs over 40 research staff. He was awarded a Professorship in 2004 and was elected a fellow of the Academy of Medical Sciences in 2008, a National Institute of Health Research Senior Investigator in 2009 and a Wellcome Trust Senior Investigator in 2011. He has published over 500 scientific papers and several books. His research interests include primary and secondary prevention of stroke, the effects of blood pressure on the brain, and the risks and benefits of aspirin.
Professor Peter Rothwell qualified in medicine from the University of Edinburgh in 1987 and after completing his early postgraduate clinical training he moved to Oxford as Clinical Lecturer in Neurology in 1996. Professor Peter Rothwell was awarded an MRC Senior Clinical Fellowship in 1999 and set up the Stroke Prevention Research Unit in 2000, which now employs over 40 research staff. He was awarded a Professorship in 2004 and was elected a fellow of the Academy of Medical Sciences in 2008, a National Institute of Health Research Senior Investigator in 2009 and a Wellcome Trust Senior Investigator in 2011. He has published over 500 scientific papers and several books. His research interests include primary and secondary prevention of stroke, the effects of blood pressure on the brain, and the risks and benefits of aspirin.
Peter is clinically active, working as a Consultant Neurologist for the Oxford University Hospitals Trust, and is Founding Director of a new purpose-built Centre for Prevention of Stroke and Dementia on the John Radcliffe Hospital site.
Clinical trials of different aspirin doses and dosing regimens
Prof. Peter Rothwell (Centre for the Prevention of Stroke and Dementia, Oxford, UK) then looked at the clinical trial data for different aspirin doses, dosing regimens and compliance with treatment in order to explain the effects this can have on the effectiveness of treatment in clinical practice. In particular, he sought to answer how we can estimate the likely effect of aspirin in clinical practice for patients who take the drug regularly.
Clinical trials of aspirin in the prevention of vascular events are quite heterogeneous and trying to come up with a simple answer to a complicated question from pooled analyses can be over-simplistic. Trials investigating aspirin’s effect on the prevention of vascular events have also evolved over time. Secondary prevention trials in the 1980s used high-dose aspirin in a mostly male smoking or ex-smoker population; early primary prevention trials were also done in men only, usually at age less than 70 years and mostly with low BMI; more recent primary prevention trials included women there were fewer smokers and a more mixed BMI profiles and the most recent primary prevention trials from 2010 onwards have tended to look at special populations with generally higher BMI such as diabetics (ASCEND), patients with a high vascular risk (ARRIVE) and older patients (ASPREE). Additionally, the wider use of statins and BP-lowering drugs has also changed the primary care prevention landscape.
The clinical trial setting is also different from clinical practice. The intention-to-treat analysis is important for limiting bias, but results can mislead due to compliance issues. This makes on-treatment analysis of some interest but this raises issues of bias and loss of randomised groups. In contrast, time-course analysis can be helpful in understanding the effects of increasing treatment drop-out and drop-in overtime during follow-up whilst still retaining the intention to treat analysis and randomised groups. Prof. Rothwell reviewed the available data on time-course analysis of the effects of aspirin in randomised trials. The data suggest that patients who take aspirin for primary prevention of CVD need to have good compliance rates in order to reap benefits over time.
Ongoing trials of different aspirin doses or dosing regimens were also reviewed. ADAPTABLE is a pragmatic trial of patients at high risk of ischaemic events. Patients are randomised to receive either 81 or 325 mg of aspirin daily in a non-blinded open-label manner. The study aims to recruit 15,000 participants with known atherosclerotic CVD enrolled over 36 months with a maximum follow up of 40 months. The primary outcomes are all-cause death and hospitalisation for MI or stroke. The primary safety endpoint is hospitalisation for a major bleeding event with a blood transfusion. The study commenced in April 2016 and aims to complete it by June 2020.
Another new study, ANDAMAN, has been designed to look at aspirin twice daily in patients with diabetes and ACS. The study will compare a dose of 100 mg enteric-coated aspirin given in the evening with a twice-daily 100 mg AM and PM dose. The primary outcome for this study is the first serious vascular event occurring within 18 months after randomisation. The study which started in 2016 is due to reach its final data collection for the primary outcome measure in February 2020.
These trials along with updated data to pre-existing analysis with recent trial data should help to produce the evidence base that dose and dosing regimen is important in terms of clinical outcomes for the antithrombotic effects of aspirin. It appears that ‘one dose fits all’ approach may not be appropriate for aspirin in the prevention of vascular events or cancer and factors such as body weight and/or BMI may need to be taken into account.