Summary of stroke guidelines
The purpose of this document is to keep a working list of international guidelines with information on aspirin and stroke. The guidelines themselves should be read for further information.
UK guidelines
Primary prevention of cardiovascular disease
NICE CKS Antiplatelet treatment for primary prevention of cardiovascular disease [Last revised August 2020] “Do not routinely prescribe antiplatelet treatment for the primary prevention of cardiovascular disease (CVD).” It then states, “Consider prescribing aspirin in people with a high risk of stroke or myocardial infarction.” The guidelines remind readers that aspirin is not licensed for the primary prevention of CVD and that people can reduce their CVD risk by other means such as smoking cessation or taking at statin. The guidelines emphasise “if aspirin is being considered, discuss the likely benefits (reduced CVD risk) and risks (such as gastrointestinal bleeding) with the person.”
For further information see:
https://cks.nice.org.uk/topics/antiplatelet-treatment/
NICE also recommend reading their advice on managing antiplatelet-induced dyspepsia:
https://cks.nice.org.uk/topics/antiplatelet-treatment/management/secondary-prevention-of-cvd/#managing-antiplatelet-induced-dyspepsia
Secondary prevention of cardiovascular disease
NICE CKS Antiplatelet treatment for the secondary prevention of CVD [last revised August 2020] suggests antiplatelet treatment for people who have had:
- Acute coronary syndrome [initially dual antiplatelets – aspirin 75 mg daily plus ticagrelor 90 mg twice daily for 12 months]
- Angina [usually low-dose aspirin 75 mg daily]
- Atrial fibrillation [AF] but notes that anticoagulants are usually prescribed
- Myocardial infarction [dual antiplatelet e.g. aspirin plus initial second antiplatelet with aspirin continued indefinitely]
- Stent implantation [aspirin 75-100 mg daily in combination with a second agent for a variable length of time depending on bleeding risk and if ACS or stable coronary artery disease and then aspirin alone].
- Stroke or TIA – in people who have had a stroke or TIA [clopidogrel is the preferred antiplatelet medication but if contraindicated give modified release [MR] dipyridamole combined with low-dose aspirin or if not able to take MR dipyridamole then use aspirin alone].
- Peripheral artery disease [clopidogrel 75 mg daily is the preferred antiplatelet but if contraindicated give low-dose aspirin]
Guidance around managing dyspepsia including Heliocobacter pylori testing and PPI usage is also included. The guidelines explain that risk factors for having a GI bleed include:
- Taking high dose aspirin
- Older age [especially over 70 years]
- History of GI bleed, ulcer or perforation
- Heliocobacter pylori infection
- Concomitant use of other medicines that increase the risk of a GI bleed
Please see
https://cks.nice.org.uk/topics/antiplatelet-treatment/management/secondary-prevention-of-cvd/
For further information about aspirin for secondary CVD prevention.
Stroke and TIA
NICE NG128 May 2019 Stroke and transient ischaemic attack in over 16s: diagnosis and initial management.
https://www.nice.org.uk/guidance/ng128/resources/stroke-and-transient-ischaemic-attack-in-over-16s-diagnosis-and-initial-management-pdf-66141665603269
states:
1.1.4. “Offer aspirin (300 mg daily), unless contraindicated, to people who have had a suspected TIA, to be started immediately.’
Further information about this decision can be found at:
https://www.nice.org.uk/guidance/ng128/resources/stroke-and-transient-ischaemic-attack-in-over-16s-diagnosis-and-initial-management-pdf-66141665603269
and
https://www.nice.org.uk/guidance/ng128/evidence/a-aspirin-pdf-6777399566
The same guidelines recommend aspirin for people with acute ischaemic stroke, as soon as possible within 24 hours, where a diagnosis of intracerebral haemorrhage has been excluded using brain imaging. For those without dysphagia give aspirin 300 mg orally and those with dysphagia can be give the same dose rectally or via an enteral tube. Aspirin 300 mg daily should be continued until long term antithrombotic treatment is agreed 2 weeks post stroke or at discharge.
A proton pump inhibitor is recommended in addition to aspirin for those with dyspepsia linked to aspirin use.
For further details on aspirin use and stroke read the full guidance.
Atrial fibrillation (AF)
NICE Atrial Fibrillation: diagnosis and management (NG196) (Published 27 April 2021) https://www.nice.org.uk/guidance/NG196 states that ‘for most people the benefit of anticoagulation outweighs the bleeding risk.’ They recommend offering anticoagulation with a direct-acting oral anticoagulant (DOAC), to adults with AF and a CHA2DS2-VASc score of 2 or more. Offer warfarin if a DOAC such as apixaban, dabigatran, edoxaban and rivaroxaban are contraindicated or not tolerated. The guidelines state that aspirin monotherapy should not be offered solely for the purposes of stroke prevention in people with AF. NICE offer guidance for antiplatelet therapy in people with a separate indication for anticoagulant therapy in their NICE (NG185) Acute coronary syndromes guidelines https://www.nice.org.uk/guidance/ng185
Prevention of cardiovascular events in diabetics
NICE NG17 2015 (updated December 2020) states “Do not offer aspirin for the primary prevention of cardiovascular disease to adults with type 1 diabetes.”
https://www.nice.org.uk/guidance/ng17/resources/type-1-diabetes-in-adults-diagnosis-and-management-pdf-1837276469701
NICE NG28 2015 (updated December 2020) Type 2 diabetes in adults- management state “Do not offer antiplatelet therapy (aspirin or clopidogrel) for adults with type 2 diabetes without cardiovascular disease.”
https://www.nice.org.uk/guidance/ng151/resources/user-guide-and-data-sources-pdf-8834927870
USA guidelines
Primary prevention of cardiovascular disease
The U.S. Preventative Services Task Force (USPSTF) was created in 1984 and is an independent, volunteer panel of national experts in preventative and evidence-based medicine. They use the following to grade their recommendations: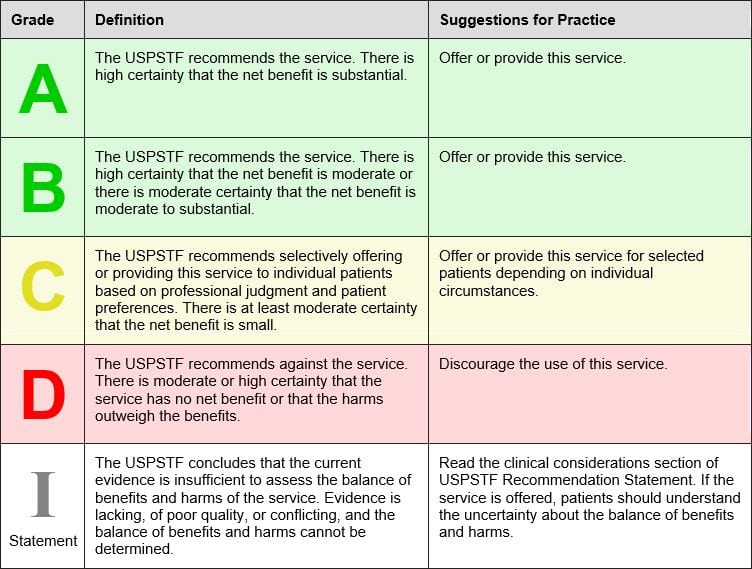
The USPSTF April 2016 recommends low-dose aspirin to prevent cardiovascular disease and colorectal cancer in the following groups:
Grade B Adults age 50 to 59 years with a 10 year CVD risk of greater than 10% who are not at increased risk for bleeding, have a life expectancy of at least 10 years and are willing to take low-dose aspirin for at least 10 years.
Grade C Adults age 60 to 69 years with a 10 year CVD risk of greater than 10% should consider their individual risks versus benefits of long term low dose aspirin. Those who are not at risk of bleeding, have a life expectancy of at least 10 years and are willing to take low-dose aspirin for at least 10 years are more likely to benefit.
“Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin.”
The USPSTF state that there is insufficient current evidence to assess the balance of benefits versus harms of initiating aspirin for primary prevention of CVD and CRC in adults younger than 50 years or 70 years or older. (Grade I).
For more information see:
The USPSTF is currently commissioning an update to its guidance on aspirin and primary prevention. More information can be found at:
The American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines on the primary prevention of cardiovascular disease (2019 ACC/AHA Guidelines on the primary Prevention of Cardiovascular Disease) states:
“Aspirin is well established for secondary prevention of [atherosclerotic cardiovascular disease] ASCVD and is widely recommended for this indication, but recent studies have shown that in the modern era, aspirin should not be used in the routine prevention of ASCVD due to lack of net benefit.”
In particular they state that aspirin should be avoided in the following groups:
- People with an increased risk of bleeding e.g. history of GI bleeding, bleeding from other sites, age> 70 years, thrombocytopenia, coagulopathy, chronic kidney disease and concurrent use of NSAIDS and anticoagulants.
AHA makes the following recommendations based on a meta-analysis of 3 recent trials:
- “Low-dose aspirin might be considered for primary prevention of ASCVD in select higher ASCVD adults aged 40-70 years who are not at increased bleeding risk
- Low-dose aspirin should not be administered on a routine basis for primary prevention of ASCVD among adults > 70 years.
- Low-dose aspirin should not be administered for primary prevention among adults at any age who are at increased bleeding risk.”
For further information see:
https://www.jacc.org/doi/pdf/10.1016/j.jacc.2019.03.010
The American Diabetes Association (ADA) (Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43(suppl.1): S111-S134) state that for the case of primary CVD prevention;
‘Aspirin therapy (75-162mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased cardiovascular risk, after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding.’
The ADA review clinical trial work including the Antithrombotic trialists’ collaboration, ASCEND, ARRIVE and ASPREE and conclude that aspirin appears to have a modest impact on decreasing ischemic vascular events especially where atherosclerotic cardio vascular disease (ASCVD) risk is higher. This however is tempered by its main side effect of increased gastrointestinal (GI) bleeding which may be as high as 5 per 1,000 per year in the real-world setting. Where ASCVD risk is more than 1% per year the number of ASCVD events that are averted are comparable to the number of GI bleeding events caused. The ADA however recognise that CVD events and GI bleeding events ‘do not have equal effects on long-term health’.
The ADA explain that men and women age 50 years or older with diabetes and one additional CVD risk factor such as family history of ASCVD, high blood pressure, high cholesterol, smoking, chronic kidney disease and who are not at increase bleeding risk e.g. older age, anaemia, renal disease, are most likely to benefit from using low-dose aspirin for the primary prevention of ASCVD. They also suggest that non-invasive imagining techniques could potentially help identify people for aspirin primary prevention therapy.
For more information see:
https://care.diabetesjournals.org/content/44/Supplement_1/S125
Secondary prevention of cardiovascular disease
The American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines on the primary prevention of cardiovascular disease 2019 guidance states;
“Aspirin is well established for secondary prevention of [atherosclerotic cardiovascular disease] ASCVD and is widely recommended for this indication.”
The AHA/ASA stoke guidelines (Guidelines for Early Management of Patients With Acute Ischemic Stroke: 2019) state:
“Administration of aspirin is recommended in patients with AIS [acute ischemic stroke] within 24-48 hours after onset. For those treated with IV alteplase, aspirin administration is generally delayed until 24 hours later but might be considered in the presence of concomitant conditions for which such treatment given in the absence of IV altephase is known to provide substantial benefit or withholding such treatment is known to cause substantial risk.”
For further information see:
https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000211
The American Diabetes Association (ADA) (Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43(suppl.1): S111-S134) recommends low-dose aspirin (75-162 mg/day) for secondary prevention in people with a history of cardiovascular disease (CVD). For those unable to take aspirin, clopidogrel (75mg/day) can be used. Dual antiplatelet therapy with low-dose aspirin and a P2Y12 inhibitor is recommended for at least a year after acute coronary syndrome therapy (ACS).
For more information see:
https://care.diabetesjournals.org/content/44/Supplement_1/S125
European guidelines
Primary prevention of cardiovascular disease
Key Message: “Antiplatelet therapy is not recommended in individuals free from CVD, due to the increased risk of major bleeding.” European Society of Cardiology (ESC) 2016
The 2016 European guidelines on CVD prevention review the evidence for antiplatelet therapy in individuals without CVD and conclude that current evidence does not support the use of aspirin in those without CVD due to the risk of a major bleed.
For more information see:
The ESC 2019 guidelines on diabetes (DM), pre-diabetes, and cardiovascular disease state:
- “Patients with DM and symptomatic CVD should be treated no differently to patients without DM
- In patients with DM at moderate CV risk, aspirin for primary prevention is not recommended
- In patients with DM at high/very high CV risk, aspirin may be considered in primary prevention
For more information see:
In addition, the ESC 2019 diabetes guidelines identify the following gaps in the evidence relating to platelet use in people with diabetes:
- Type 1 diabetes and CVD prevention [in vivo platelet activation has been reported]
- Body mass and antiplatelet responsiveness especially in those with diabetes and obesity where higher dose strategies need investigation
- Are antithrombotic effects similar in pre-DM and DM?
The ESC does not recommend aspirin for healthy people over 70 following results of the ASPREE trial.
The ESC state in a press release following results of ARRIVE that the benefits of aspirin in primary prevention remain unclear.
In another press release following the ASCEND trial the ESC state that the bleeds and benefits are balanced in people with diabetes using aspirin for primary CVD prevention and that no cancer benefit was found in this study.
Secondary prevention of cardiovascular disease
The 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes recommended aspirin for CVD event prevention at a dose of 75-100 mg daily in patients with previous MI or revascularization. In addition, aspirin 75-100 mg daily can also be considered for people without a history of MI or revascularization but where there is evidence of coronary artery disease using imaging tests.
For further information see:
https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Chronic-Coronary-Syndromes
The 2016 European Guidelines for CVD prevention in clinical practice make the following recommendations for antiplatelet therapy (see table below). This is a based on a careful appraisal of risks versus benefits of aspirin or other antiplatelet therapy. The guidelines state that there is a gap in the evidence concerning new antiplatelet drugs in patients with stable coronary artery disease as well as a gap in the understanding about the use of new antiplatelet drugs used in combination with anticoagulation treatment.
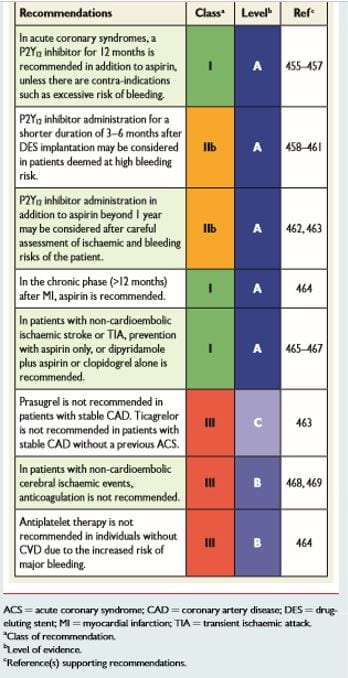
Source 2016 European guidelines for cardiovascular disease prevention
For further information please see the full guidance at:
Chinese guidelines
Primary prevention of cardiovascular disease
Cardiovascular disease (CVD) is a growing health burden in China and primary prevention strategies are important because over 70% of those experiencing a first Coronary Heart Disease (CHD) event in China died outside of the hospital setting1.
Primary prevention of CVD in China is a top priority, in particular lifestyle factors such as smoking, diet, weight and increasing activity levels in order to reduce risk factors such as high blood pressure, high cholesterol and control diabetes2.
CVD risk assessment is an important way to identify those at high-risk of CVD in the Chinese population without current CVD. Specific risk assessment tools for the Chinese population have been developed based the Chinese Multi –provincial cohort study.
The 2012 China National Plan for Non-Communicable diseases [NCD] prevention and treatment3 has clear targets for CVD prevention. The 2016 Chinese guidelines of dyslipidemia management and the 2017 Chinese guidelines for CVD prevention have new recommendations for risk assessment in China.
The 2016 China expert consensus advocates that for individuals with a ten year arteriosclerotic cardiovascular disease (ASCVD)4 risk of greater than or equal to 10%, aspirin should be considered for the primary prevention of CVD.
In the 2020 Chinese guidelines5 for the prevention of cardiovascular diseases low-dose aspirin (75-100 mg daily) can be considered in adults aged 40–70 years who are at high ASCVD risk but not at increased bleeding risk, with at least one risk-enhancing factor (e.g. CAC >100, carotid plaque, family history of premature CVD, poor control of cholesterol, blood pressure and glucose), with level A, class IIb recommendations.
‘ Prophylactic use of low-dose aspirin is a treatment strategy for patients with high-risk ASCVD and enhancement factors.’
Chinese Society of Cardiology of Chinese Medical Association 2020
Bleeding risk factors include:
• Previous gastrointestinal bleeding or peptic ulcer disease
• History of major bleeding
• Low body weight
• Age > 70 years
• Thrombocytopenia
• Coagulation dysfunction
• Chronic kidney disease
• Use of other medicines that increase bleeding risk (e.g. NSAIDS, steroids, non-vitamin K antagonists, oral anticoagulants, warfarin).
A proton pump inhibitor can be considered to reduce bleeding risk.
For further information see:
The 2019 Chinese expert consensus statement6 on aspirin application in primary prevention of CVD recommend assessing four measures before considering aspirin:
1. Benefit versus bleeding risk ratio; screen and exclude those at high risk of bleeding and reassess periodically or dynamically during aspirin treatment.
2. Treat gastrointestinal active pathological changes in advance e.g. eradicate Helicobacter pylori and consider prophylaxis with a proton pump inhibitor or H2 receptor antagonist.
3. Adhere to a healthy lifestyle and control blood pressure (BP), blood sugar and lipid levels. Consider aspirin in people with a BP < 140/90 mmHg.
4. Communicate and obtain consent for aspirin use from the patient
The consensus statement recommends the ASCVD high-risk groups for consideration of low-dose aspirin (75–100 mg per day) for primary CVD prevention:
• Adults aged 40–69 years, with a 10-year expected risk of ASCVD is ≥10% at initial risk assessment with ≥3 major risk factors that are poorly controlled after treatment intervention.
• The main risk factors include: hypertension, diabetes, dyslipidemia (TC ≥6.2 mmol/L, or LDL ≥4.1 mmol/L, or HDL <1.0 mmol/L), smoking, family history of early onset CVD, obesity, CAC score ≥100, or coronary artery stenosis <50%. (Coronary imaging of patients for primary CVD prevention is not routinely recommended).
Aspirin is not recommended for primary CVD prevention in people who have a higher risk of bleeding. These include:
• Those using drug with a bleeding risk (e.g. anti- platelets, anti-coagulants, hormones, NSAID)
• Gastrointestinal bleeding, peptic ulcer or history of bleeding in other sites
• Age 70 years and over
• Thrombocytopenia
• Coagulopathy
• Severe liver disease
• CKD stage 4-5
• Uneradicated H.pylori infection
• Uncontrollable hypertension
Or in those whose risk of bleeding is greater than their risk of thrombosis. The consensus statement includes a useful flow chart for population screening for aspirin primary CVD prevention usage.
For further information please see:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7249706/
In the 2017 Chinese CVD prevention guide7, low dose aspirin is recommended for: 10-year ASCVD risk ≥10%; Diabetic patients, age ≥50 years, with at least one major risk factor: Hypertensive patients with BP <150/90 mmHg, accompanied by ≥3 major risk factors; patients with CKD (eGFR 30–45 mL/min/1.73 m2 ; patients other than above with ≥4 risk factors.
References
1.Chin J Cardiol, March 2012; Vol 40 No 3.
2. Walker J, Hutchison P, Ge J et al 2018 Aspirin:120 years of innovation. A report from the 2017 Scientific Conference of the International Aspirin Foundation. ecancer 12 813 https://doi.org/10.3332/ecancer.2018.813
3.China National Plan for NCD Prevention and Treatment (2012-2015). http://www.chinaede.en.en [accessed June 14, 2014].
4.ASCVD http://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate
5. Chinese Society of Cardiology of Chinese Medical Association; Cardiovascular Disease Prevention and Rehabilitation Committee of Chinese Association of Rehabilitation Medicine; Cardiovascular Disease Committee of Chinese Association of Gerontology and Geriatrics; Thrombosis Prevention and Treatment Committee of Chinese Medical Doctor Association. [Chinese guideline on the primary prevention of cardiovascular diseases]. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:1000–38
6.Li XY, Shi ZW, Zhao D, Yin DW; writing group of 2019 Chinese expert consensus statement on aspirin application in primary prevention of cardiovascular disease. 2019 Chinese expert consensus statement on aspirin application in primary prevention of cardiovascular disease. Chin Med J (Engl) 2020;133:1221–3.
7.Writing Group of Chinese Cardiovascular Disease Prevention Guide (2017), Editorial Board of Chinese Journal of Cardiovascular Diseases. Chinese cardiovascular disease prevention guide (2017). Chin J Cardiovasc Dis 2018;46:10–25.
Secondary prevention of stroke prevention in China
The Chinese clinical guidelines for the secondary prevention of ischemic stroke and TIA recommend an optimal dosage of aspirin between 75 and 150 mg/day. A combination of aspirin and clopidogrel for 21 days is recommended for patients with minor stroke or high-risk TIA within 24 hours of onset1. These recommendations are based on the CHANCE trial2.
The 2016 Chinese expert consensus statement3 explains that atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disease burden for China. Aspirin is very useful for China’s large population due to its availability and relative effectiveness. The 2016 expert consensus group makes recommendations for aspirin use in stable coronary artery disease, acute coronary syndrome, transient ischemic attack, stroke and peripheral artery disease.
Chinese Society of Cardiology and Chinese College of Cardiovascular Physicians Expert Consensus4 statement on dual antiplatelet therapy in patients with coronary artery disease is another resource [for Chinese speaking readers].
References CVD prevention
1. Wang Y, Liu M and Pu C 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke 2017; 12(3):302-320.
2.Wang Y, Pan Y, Zhao X et al Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE) Trial: One-Year outcomes [j]. Circulation 2015; 132(1):40-46.
3. Aspirin use in patients with atherosclerotic cardiovascular disease: the 2016 Chinese expert consensus statement. Zhonghua Nei Ke Za Zhi. 2017 Jan;56(1):68-80 doi: 10.3760/cma.j.issn.0578-1426.2017.01.020
4. Chinese Society of Cardiology and Chinese College of Cardiovascular Physicians Expert Consensus statement on dual antiplatelet therapy in patients with coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi. 2021 May 24;49(5):432-454. doi:10.3760/cma.j.cn112148-20210125-00088











