Summary of USA guidelines for aspirin
August 2023
The purpose of this document is to keep a working list of USA guidelines with information on aspirin. The guidelines themselves should be read for further information.
USA guidelines for VTE prevention in surgical patients make the following recommendations involving aspirin:
- In patients undergoing total hip or total knee arthroplasty, conditional recommendations included using either aspirin or anticoagulants, as well as for a direct oral anticoagulant over low-molecular-weight heparin (LMWH).
Primary prevention of cardiovascular disease
The U.S. Preventative Services Task Force (USPSTF) was created in 1984 and is an independent, volunteer panel of national experts in preventative and evidence-based medicine. They use the following to grade their recommendations:
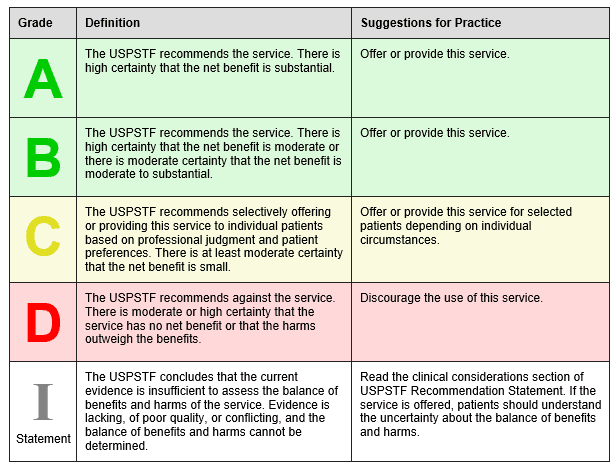
The USPSTF April 2016 recommends low-dose aspirin to prevent cardiovascular disease and colorectal cancer in the following groups:
Grade B Adults age 50 to 59 years with a 10 year CVD risk of greater than 10% who are not at increased risk for bleeding, have a life expectancy of at least 10 years and are willing to take low-dose aspirin for at least 10 years.
Grade C Adults age 60 to 69 years with a 10 year CVD risk of greater than 10% should consider their individual risks versus benefits of long term low dose aspirin. Those who are not at risk of bleeding, have a life expectancy of at least 10 years and are willing to take low-dose aspirin for at least 10 years are more likely to benefit.
“Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin.”
The USPSTF state that there is insufficient current evidence to assess the balance of benefits versus harms of initiating aspirin for primary prevention of CVD and CRC in adults younger than 50 years or 70 years or older. (Grade I).
https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer
The USPSTF is currently commissioning an update to its guidance on aspirin and primary prevention. More information can be found at:
https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/aspirin-use-to-prevent-cardiovascular-disease-and-colorectal-cancer-preventive-medication
The American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines on the primary prevention of cardiovascular disease (2019 ACC/AHA Guidelines on the primary Prevention of Cardiovascular Disease)
states:
“ Aspirin is well established for secondary prevention of [atherosclerotic cardiovascular disease] ASCVD and is widely recommended for this indication, but recent studies have shown that in the modern era, aspirin should not be used in the routine prevention of ASCVD due to lack of net benefit.”
In particular they state that aspirin should be avoided in the following groups:
- People with an increased risk of bleeding e.g. history of GI bleeding, bleeding from other sites, age> 70 years, thrombocytopenia, coagulopathy, chronic kidney disease and concurrent use of NSAIDS and anticoagulants.
AHA makes the following recommendations based on a meta-analysis of 3 recent trials:
- “Low-dose aspirin might be considered for primary prevention of ASCVD in select higher ASCVD adults aged 40-70 years who are not at increased bleeding risk
- Low-dose aspirin should not be administered on a routine basis for primary prevention of ASCVD among adults > 70 years.
- Low-dose aspirin should not be administered for primary prevention among adults at any age who are at increased bleeding risk.”
For further information see:
https://www.jacc.org/doi/pdf/10.1016/j.jacc.2019.03.010
The American Diabetes Association (ADA) (Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43(suppl.1): S111-S134) state that for the case of primary CVD prevention;
‘Aspirin therapy (75-162mg/day) may be considered as a primary prevention strategy in those with diabetes who are at increased cardiovascular risk, after a comprehensive discussion with the patient on the benefits versus the comparable increased risk of bleeding.’
The ADA review clinical trial work including the Antithrombotic trialists’ collaboration, ASCEND, ARRIVE and ASPREE and conclude that aspirin appears to have a modest impact on decreasing ischemic vascular events especially where atherosclerotic cardio vascular disease (ASCVD) risk is higher. This however is tempered by its main side effect of increased gastrointestinal (GI) bleeding which may be as high as 5 per 1,000 per year in the real-world setting. Where ASCVD risk is more than 1% per year the number of ASCVD events that are averted are comparable to the number of GI bleeding events caused. The ADA however recognise that CVD events and GI bleeding events ‘do not have equal effects on long-term health’.
The ADA explain that men and women age 50 years or older with diabetes and one additional CVD risk factor such as family history of ASCVD, high blood pressure, high cholesterol, smoking, chronic kidney disease and who are not at increase bleeding risk e.g. older age, anaemia, renal disease, are most likely to benefit from using low-dose aspirin for the primary prevention of ASCVD. They also suggest that non-invasive imagining techniques could potentially help identify people for aspirin primary prevention therapy.
For more information see:
https://care.diabetesjournals.org/content/44/Supplement_1/S125
Primary prevention of colorectal cancer
The USPSTF recommends low-dose aspirin for the prevention of colorectal cancer in certain groups (see primary prevention of CVD above).
The USPSTF is currently commissioning an update to its guidance on aspirin and primary prevention. More information can be found at:
https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/aspirin-use-to-prevent-cardiovascular-disease-and-colorectal-cancer-preventive-medication
Secondary prevention of cardiovascular disease
The American College of Cardiology (ACC) and American Heart Association (AHA) Guidelines on the primary prevention of cardiovascular disease 2019 guidance states;
“Aspirin is well established for secondary prevention of [atherosclerotic cardiovascular disease] ASCVD and is widely recommended for this indication.”
The ACC/AHA in their 2014 Non ST-Elevation acute coronary syndromes guidelines recommend aspirin as soon as possible before percutaneous intervention (PCI) and then “after PCI, aspirin should be continued indefinitely at a dose of 81 mg to 325 mg daily.”
For further information see:
https://www.jacc.org/doi/pdf/10.1016/j.jacc.2014.09.017
The AHA/ASA stoke guidelines (Guidelines for Early Management of Patients With Acute Ischemic Stroke: 2019) state:
“Administration of aspirin is recommended in patients with AIS [acute ischemic stroke] within 24-48 hours after onset. For those treated with IV alteplase, aspirin administration is generally delayed until 24 hours later but might be considered in the presence of concomitant conditions for which such treatment given in the absence of IV altephase is known to provide substantial benefit or withholding such treatment is known to cause substantial risk.”
For further information see:
https://www.ahajournals.org/doi/epub/10.1161/STR.0000000000000211
The American Diabetes Association (ADA) (Cardiovascular disease and risk management: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43(suppl.1): S111-S134) recommends low-dose aspirin (75-162 mg/day) for secondary prevention in people with a history of cardiovascular disease (CVD). For those unable to take aspirin, clopidogrel (75mg/day) can be used. Dual antiplatelet therapy with low-dose aspirin and a P2Y12 inhibitor is recommended for at least a year after acute coronary syndrome therapy (ACS).
For more information see:
https://care.diabetesjournals.org/content/44/Supplement_1/S125
Aspirin and pre-eclampsia
The International Federation of Gynecology and Obstetrics recommend that women identified as high risk of pre-eclampsia during first trimester screening should be given aspirin prophylaxis (150mg at night from 11-14 weeks gestation until delivery or the diagnosis of pre-eclampsia). They do not advocate a policy of low-dose aspirin for all pregnant women.
Poon LC, Shennan A, Hyett JA et al The International federation of gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynecol Obstet 2019; 145 (Suppl.1) 1-33. @
https://obgyn.onlinelibrary.wiley.com/doi/epdf/10.1002/ijgo.12802
The U.S. Preventative services Task Force (USPSTF) recommends (Grade B) low-dose aspirin (81 mg per day) after 12 weeks gestation as a preventative medication in women at high risk of pre-eclampsia6.
USPSTF Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventative medication. 2014 (currently being updated) available @ https://www.uspreventativeservicestaskforce.org/uspstf/recommendations/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortlality-from-preeclampsia-preventative-medication











